Why Study Brackish Groundwater?
Sources of Dissolved Solids in Brackish Groundwater
How is Brackish Groundwater Being Used?


Sources of Dissolved Solids in Brackish Groundwater
A variety of conditions and mechanisms can cause groundwater to become brackish. An understanding of the sources of dissolved solids that contribute to the formation of brackish groundwater can help determine where brackish aquifers are likely to exist and can provide clues about other characteristics, such as the chemical composition, of brackish aquifers.
Dissolution of minerals in the saturated and unsaturated zones

Salt deposits, such as halite or gypsum, are found in many sedimentary basins in the United States and are highly soluble. Other deposits are less soluble but can contribute dissolved solids when in contact with water over longer periods of time.
Salt deposits (Gypsum).
(Source: Siim Sepp, http://www.sandatlas.org/)
Connate seawater and (or) dissolution of marine sediments
|
|
"Connate" water is water that was trapped in the sediments as they were deposited. Large parts of the United States have been covered with seawater in previous geologic time periods, and geologic systems that have not been flushed by freshwater can still contain connate seawater. Sediments deposited in a marine environment, such as carbonates, can dissolve in a groundwater system. |
Movement of saline or brackish groundwater from adjacent aquifers
Saline or brackish groundwater from adjacent aquifers can mix with fresh water to create brackish conditions. Well boreholes that are open to both types of water can facilitate mixing in the subsurface. In addition, pumping wells may cause water to flow from a saline aquifer to a fresh aquifer.
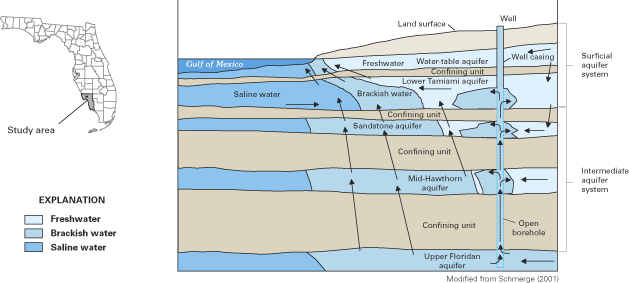
Generalized ground-water flow patterns in coastal Lee and western Collier Counties, southwestern Florida. The figure illustrates some of the paths by which brackish and saline waters migrate in the multilayered system, including natural upward leakage between aquifers, brackish-water flow in a well with an open borehole, and lateral intrusion from the Gulf of Mexico.
(Source: USGS Circular 1262)

Development of geothermal saline groundwater into a warm-spring spa.
(Source: State of Florida Department of Natural Resources. Special Publication No. 21, 1977)
Leaching from saline soils or road salt
 Salts at the land surface can leach to shallow groundwater. Evapotranspiration can concentrate dissolved salts in the soil where it subsequently can infiltrate to shallow groundwater, especially where precipitation rates are low. In addition, millions of tons of salt are applied to roads each winter. Runoff containing dissolved road salt can eventually reach shallow groundwater. In many cases, these sources of dissolved solids are associated with localized brackish groundwater and will not be studied as part of the National Brackish Groundwater Assessment
Salts at the land surface can leach to shallow groundwater. Evapotranspiration can concentrate dissolved salts in the soil where it subsequently can infiltrate to shallow groundwater, especially where precipitation rates are low. In addition, millions of tons of salt are applied to roads each winter. Runoff containing dissolved road salt can eventually reach shallow groundwater. In many cases, these sources of dissolved solids are associated with localized brackish groundwater and will not be studied as part of the National Brackish Groundwater Assessment
Salt impacted field.
(Source: Colorado State University Extension, Fact Sheet No. 0.521)
Seawater intrusion
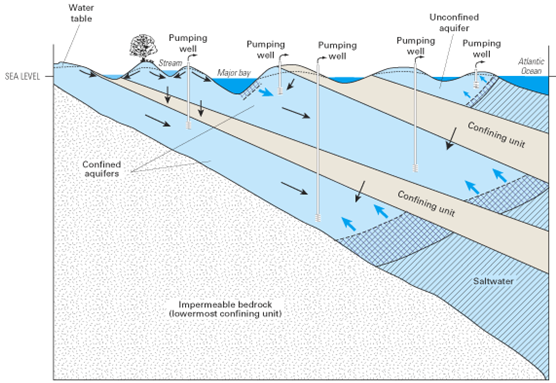 Seawater can mix with fresh water where aquifers along the coast are connected with the ocean. Pumping wells near the coast can further increase flow of seawater into fresh aquifers.
Seawater can mix with fresh water where aquifers along the coast are connected with the ocean. Pumping wells near the coast can further increase flow of seawater into fresh aquifers.
Schematic illustration of some of the modes of saltwater intrusion in a multilayer, regional aquifer system caused by ground-water pumping at wells. Saltwater moves into the unconfined aquifer from the Atlantic Ocean and into the shallow part of the top confined aquifer from the major bay. The two freshwater-saltwater interfaces at the seaward boundary of each of the confined aquifers also move landward as saltwater is drawn inland from offshore areas.
(Source: USGS Circular 1262)
Injection, leakage, or infiltration of brine from oil and gas wells
 Brine is water that contains very large amounts of dissolved salts (total dissolved-solids concentration greater than 35,000 milligrams per liter), and is a byproduct of pumping oil and gas. Most brine is injected back into the subsurface, and the rest is disposed at the land surface. Naturally fresh groundwater can mix with brine and produce brackish groundwater.
Brine is water that contains very large amounts of dissolved salts (total dissolved-solids concentration greater than 35,000 milligrams per liter), and is a byproduct of pumping oil and gas. Most brine is injected back into the subsurface, and the rest is disposed at the land surface. Naturally fresh groundwater can mix with brine and produce brackish groundwater.
Unlined brine pit on the shore of Skiatook Lake, Okla.
(Source: USGS Toxic Substances Hydrology Program)